Using CGM to Reduce Low Glucose Events
Written by: Beyond Type 2 Editorial Team
5 minute read
November 21, 2019
Always a little dizzy or shaky? Your blood sugar may be low. Read our article on how a CGM could help you reduce low blood glucose episodes.
This content was sponsored by Abbott, the makers of FreeStyle Libre 2,—a Founding Partner of Beyond Type 2.
Looking out for lows
Hyperglycemia, also called high glucose, tends to be the focus when it comes to the treatment of type 2 diabetes. It is not unusual for the diagnosis of type 2 diabetes to be made due to high glucose values and/or elevated A1c levels. For many people diagnosed with type 2 diabetes, the goal is lowering glucose to a range of 3.9-10.0 mmol/L70-180 mg/dL.¹
At the other end of the spectrum is low glucose, or hypoglycemia, which is commonly defined as glucose values below 3.9 mmol/L70 mg/dL.² Hypoglycemia can be dangerous and requires immediate attention to treat. It is important to understand when or why it can happen and work to prevent it. Hypoglycemia among people with type 2 diabetes is most commonly experienced by those on insulin therapy—but can also occur with some oral medications, even when used without insulin. If left untreated, severe hypoglycemia (<3.0 mmol/L54 mg/dL) can lead to unconsciousness, coma and even death.
People experience many different symptoms with hypoglycemia; a few include confusion, dizziness and feeling shaky. Not all people feel hypoglycemia symptoms the same way, and some people with hypoglycemia unawareness may not feel any symptoms at all.³
The CGM Experience
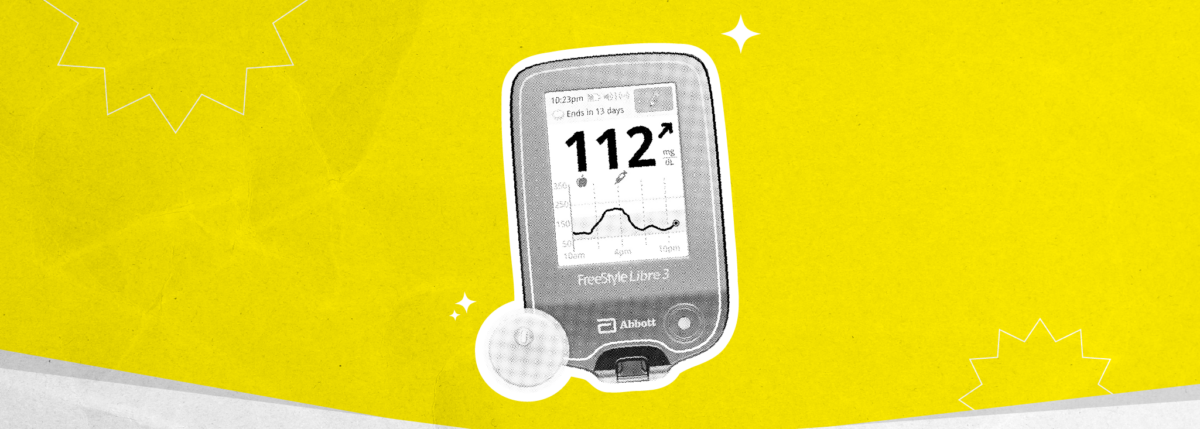
Fortunately, for people living with type 2 diabetes, continuous glucose monitors (CGMs), such as the FreeStyle Libre 14 day system are now available for use in their self-management. CGM systems track blood glucose levels continuously and show patterns and trends over a typical day to help people better manage their diabetes.
The FreeStyle Libre 14 day sensor is worn on the back of the upper arm and tracks blood glucose levels for up to 14 days. Each scan of the sensor with either a reader or a smartphone* provides a current blood glucose reading, a trend arrow and an eight-hour history. The trend arrow can show if the glucose levels are steady, going up or going down, and at what rate the levels are changing. This allows the user to see the impact that exercise, food, medications and other factors such as stress have on blood glucose levels in real time.
The FreeStyle Libre 14 day system provides additional support in letting you know about hypoglycemia or impending hypoglycemia by displaying a ”Low Glucose” or “Glucose Going Low” message when the sensor is scanned and glucose is lower than 3.9 mmol/L70 mg/dL or projected to be lower than 3.9 mmol/L70 mg/dL within 15 minutes. The system also provides support in identifying patterns of hypoglycemia. The ‘low glucose events’ view shows the total number of low blood glucose events for a given time period and what time of day they occurred.
According to a study, patients with type 2 diabetes taking multiple daily insulin injections and using the FreeStyle Libre 14 day system reduced their time in hypoglycemia by 43 percent overall, and 54 percent percent at night. 4 Patients with type 2 also experienced 28 percent fewer episodes of hypoglycemia4 and were also able to sustain reductions over a six-month period. 4,5 Additionally, more frequent scanning was associated with less time in hypoglycemia, better time-in-range and better glucose management. 6
Insights into hypoglycemia patterns over time can empower CGM users and their healthcare providers to make changes in medication or lifestyle to achieve better diabetes management. To learn more about the benefits of the FreeStyle Libre 14 day system, click here.
* The FreeStyle LibreLink app is compatible with NFC-enabled smartphones running Android OS 5.0 or higher and iPhone 7 or later running iOS 11 or later. Use of the FreeStyle LibreLink app requires registration with LibreView, a service provided by Abbott and Newyu, Inc.
The FreeStyle LibreLink app and FreeStyle Libre 14 Day reader have similar but not identical features. Fingersticks are required for treatment decisions when you see Check Blood Glucose symbol, when symptoms do not match system readings, when you suspect readings may be inaccurate, or when you experience symptoms that may be due to high or low blood glucose. When using FreeStyle LibreLink app, access to a blood glucose monitoring system is required as the app does not provide one.
Reference:
1. Battelino, T. et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care, 2019 Aug; 42(8): 1593-1603. 2. Seaquist, E. et al. Hypoglycemia and Diabetes: A Report of a Workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care. 2013 May; 36(5): 1384–1395. 3. de Galan BE, Schouwenberg BJ, Tack CJ, Smits P. Pathophysiology and management of recurrent hypoglycaemia and hypoglycaemia unawareness in diabetes. Neth J Med. 2006;64:269-279. 4. Haak, Thomas, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicentre, open-label randomised controlled trial. Diabetes Therapy 8.1 (2017): 55-73. 5. Bolinder, Jan, et al. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. The Lancet 388.10057 (2016): 2254-2263. 6. “1Dunn, T., et al. Real-world flash glucose monitoring patterns and associations between self-monitoring frequency and glycemic measures: A European analysis of over 60 million glucose tests. Diabetes Research and Clinical Practice; 137(2018) 37-46
Indications and Important Safety Information
The FreeStyle Libre 14 day Flash Glucose Monitoring System is a continuous glucose monitoring (CGM) device indicated for the management of diabetes in persons age 18 and older. It is designed to replace blood glucose testing for diabetes treatment decisions. The System detects trends and tracks patterns aiding in the detection of episodes of hyperglycemia and hypoglycemia, facilitating both acute and long-term therapy adjustments. Interpretation of the System readings should be based on the glucose trends and several sequential readings over time. The System is intended for single patient use and requires a prescription.
CONTRAINDICATIONS: Remove the sensor before MRI, CT scan, X-ray, or diathermy treatment.
WARNINGS/LIMITATIONS: Do not ignore symptoms that may be due to low or high blood glucose, hypoglycemic unawareness, or dehydration. Check sensor glucose readings with a blood glucose meter when Check Blood Glucose symbol appears, when symptoms do not match system readings, or when readings are suspected to be inaccurate. The FreeStyle Libre 14 day system does not have alarms unless the sensor is scanned and the system contains small parts that may be dangerous if swallowed. The FreeStyle Libre 14 day system is not approved for pregnant women, persons on dialysis, or critically-ill population. Sensor placement is not approved for sites other than the back of the arm and standard precautions for transmission of blood borne pathogens should be taken. The built-in blood glucose meter is not for use on dehydrated, hypotensive, in shock, hyperglycemic-hyperosmolar state, with or without ketosis, neonates, critically-ill patients, or for diagnosis or screening of diabetes. When using FreeStyle LibreLink app, access to a blood glucose monitoring system is required as the app does not provide one. Review all product information before use or contact Abbott Toll Free (855-632-8658) or visit http://www.freestylelibre.us for detailed indications for use and safety information.
For full indications for use and safety information, see more here.

Author
Beyond Type 2 Editorial Team
This piece was authored collaboratively by the Beyond Type 2 Editorial Team. Members of that team include Project Manager T'ara Smith, Editorial Manager Todd Boudreaux, Program Manager Mariana Gómez, Director of Brand Communications Dana Howe and Editorial Associate Jordan Dakin.
Related Resources

Managing diabetes can be challenging, but new technology can help. This tip sheet highlights how...
Read more

Editor's note: This guide is meant as a recommendation, not medical advice. CGM data should...
Read more

🌟 Beyond Type 2's Virtual Summit! 🌟 Watch the recording of our first Type 2...
Read more