Closed Loop Updates: Omnipod Horizon and Medtronic 780G data presented at ADA 2020
On June 12, data from four new clinical trials of hybrid closed loop systems was presented in a landmark session at ADA 2020. These presentations come just days after Medtronic announced that their next generation 780G system had received CE Marking (the European equivalent of FDA approval) and is expected to begin shipping this fall in some European countries.
Medtronic 780G trial results
Editor’s Note: All studies referenced the Medtronic 780G system as Advanced Hybrid Closed Loop (AHCL)—We have used 780G for clarity.
The first study highlighted results from the pivotal trial of Medtronic’s next-generation 780G system. Bruce Bode, from Atlanta Diabetes Associates presented the results of the single arm, 16-site trial that enrolled 157 participants: 118 adults (ages 22-75 years) and 39 adolescents (ages 14-21 years). Inclusion criteria were that all participants were previously on pump therapy, with or without a CGM for at least 6 months.
A significant aspect of this study was testing two different basal set points, one at 6.7 mmol/L120 mg/dL and one at 5.5 mmol/L100 mg/dL. The basal set point is the target that basal insulin adjustments will be made to aim toward. A set point of 5.5 mmol/L100 mg/dL is lower than any available set point for Hybrid Closed Loop systems currently approved by the FDA.
A few key results from this study:
- Mean A1C improved across the group from 7.5 percent at baseline to 7 percent
- The percentage of participants who achieved 70 percent Time in Range (TIR) (3.9 mmol/L-10.0 mmol/L70 mg/dL-180 mg/dL) or better improved from 54 percent at baseline to 73 percent overall, and 79 percent for those using a set point of 5.5 mmol/L100 mg/dL.
- Among adolescents the number of participants hitting 70 percent Time in Range (TIR) rose from 18 percent at baseline to 59 percent overall, and 62 percent for those using a set point of 5.5 mmol/L100 mg/dL
- Overall TIR went from 68.8 percent to 74.5 percent on 780G. That number went from 71.2 percent up to 81.5 percent during overnight hours (12am-6am.)
“The results of the study are exciting and are a welcome addition to what we have seen with other advancements in automated insulin delivery systems. Most notable is the additional target blood glucose level of 5.5 mmol/L100 mg/dL—which will be lower than the other commercially available devices in this category and likely contributed to the demonstrated improvements in study outcomes, namely Time in Range and A1C,” said Dr. Anders Carlson, medical director of the Park Nicollet International Diabetes Center (IDC) in Minneapolis, Minnesota and investigator of the study.
The second speaker was Dr. Richard M Berganstal presenting results from the Fuzzy Logic Automated Insulin Regulation (FLAIR) Study which directly compared the 670G to the 780G (AHCL) system. This 90-day trial across seven international sites enrolled 113 adolescents and young adults (ages 14-29).

In addition to the lower set point option, 780G also adds automatic correction boluses, which the 670G system does not. 780G will also begin to correct over 6.7 mmol/L120 mg/dL, whereas 670G corrected over 8.3 mmol/L150 mg/dL, and the 780G no longer exits auto mode in cases of hyperglycemia.
The results showed that 780G improved outcomes over 670G in all measures
- TIR went from 57 percent at baseline to 63 percent on 670G, increasing again to 67 percent on 780G, with people spending less time in hyperglycemia on the newest system.
- Percentage of those hitting >70 percent TIR went from 12 percent at baseline to 22 percent on 670G and increased to 32 percent on 780G, with fewer severe hypos on 780G.
- Baseline average A1C levels went from 7.9 percent to 7.6 percent with 670G down to 7.4 percent with 780G.
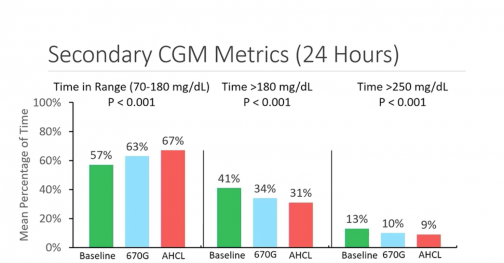
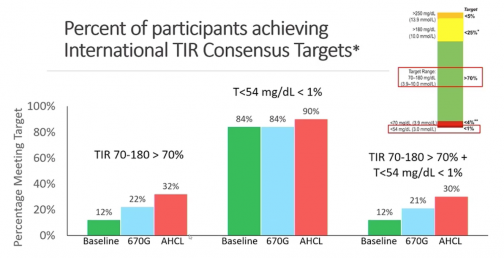
Both systems were found to be safe when evaluating events of severe hypoglycemia or diabetic ketoacidosis (DKA). Another significant data point was that the time spent in auto mode was significantly higher on 780G, 86 percent of the time, compared to 75 percent for 670G. This amounted to an average of 5.7 exits of auto mode per week on 670G and only 1.7 exits per week on 780G by comparison.
A third study from New Zealand comparing Medtronic 780G to predictive low blood glucose suspend was presented by Dr. Martin de Bock from the University of Otago. Participants in this study stayed in auto mode 96.4 percent of the time, with an average of 1.2 exits per week. De Bock confirmed what the previous studies showed regarding the efficiency of a lower set point, saying “If you were to run this in your clinic, you should start at the 5.5 mmol/L100 mg/dL [basal correction point].”
Omnipod 5, Powered by Horizon pre-pivotal trial results
After the conclusion of the three presentations on Medtronic’s 780G system, Dr. Bruce Buckingham from Stanford University presented the pre-pivotal trial results from Insulet’s Omnipod 5, powered by Horizon. These are the first outpatient results the public has seen.
As a reminder, the Omnipod 5 (previously Omnipod Horizon) algorithm lives on the pod itself and communicates directly to a Dexcom G6. It will be controlled via an app on your Samsung smartphone device or via a locked down touchscreen Personal Diabetes Manager (PDM). The Omnipod does not need to stay in range of a device like current DIY Loop systems to operate in hybrid closed loop mode.
The pre-pivotal study had 36 participants, 18 children (ages 6-13) and 18 adults (ages 14-70). Objectives were to assess the safety and effectiveness of the system at higher blood glucose targets of 7.2-8.3 mmol/L130-150 mg/dL as well as giving the patients free choice of target ranges from 6.1-8.3 mmol/L110-150 mg/dL.
Omnipod 5 improved outcomes across the board, with the biggest improvements coming when patients were given the choice to customize their own blood glucose targets. During this “free choice” testing over four-to-nine weeks, TIR increased from 65.6 percent in adults to 73.8 percent. Among children TIR increased from 51 percent to 70.1 percent. This amounted to 17/18 children achieving >60 percent TIR compared to just 4/18 on standard therapy.
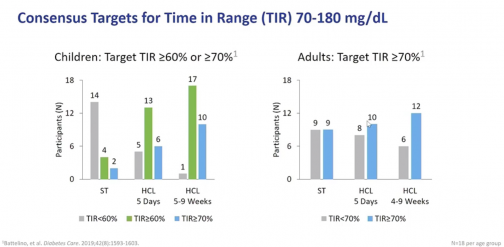
After announcing a pause in the Omnipod 5 pivotal trial in March due to a rare software anomaly, Insulet announced just last week that the trial is back underway. The next generation device is expected to be available in the United States in the first half of 2021.
Click here for complete coverage of ADA 2020 from Beyond Type 2.





