News From DiabetesMine™ D-Data Exchange 2020
The DiabetesMine™ D-Data ExChange is a biannual event where diabetes advocates, pharma leaders, developers, clinicians, designers and other groups interested in diabetes tech for diabetes management, and of course a better quality of life, get together. Due to the COVID-19 pandemic, this year’s 2020 summer event is virtual.
An event where people with diabetes can be heard, D-Data began in 2013 as a spinoff the DiabetesMine Innovation Summit and started the global #WeAreNotWaiting movement, which celebrates and recognizes DIY patient entrepreneurship. Because of D-Data, we’ve seen a rise in game-changing contributions to the diabetes industry by companies and organizations like the Nightscout Foundation, Tidepool and DIY solutions.
Discover, Learn and Try
At the DiabetesMine™ D-Data ExChange, participants can discover, learn about and try demos of new diabetes tech, some of which may be already be approved or going through the U.S. Food and Drug Administration (FDA) approval process. Take a look below to read about the latest diabetes innovations on the horizon from AI apps for parents of children with diabetes to non-invasive continuous glucose monitoring for people with prediabetes and type 2 diabetes, plus new learnings about automated insulin delivery (AID) systems for the diabetes community.
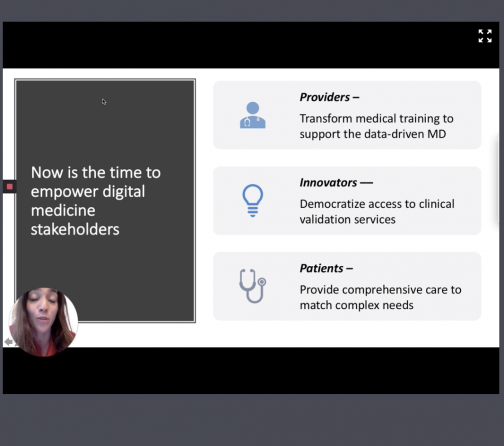
Data-Driven Care for All: Challenges & Opportunities” by Sarah Zweifach, NYU, Clinical Product Strategy & Digital
Health
This is the best time to get digital tools for patients. What are our first steps for innovation? Collaboration needs to be the first step. The participation of the three major stakeholders is needed: providers, patients and innovators.
Most medical curricula haven’t changed much in the last couple of decades.” Sarah remarks on the lack of training on the use of telemedicine for medical students. Only 19 percent of students felt prepared to use technology to care for patients.
In 2019, 38 percent of health systems CEOs didn’t have a digital health strategy. However, Zweifach acknowledged that the coronavirus pandemic has solidified telemedicine as a mainstay in healthcare. In fact, when asked what barriers prevented providers from using telemedicine over the years, she responded that regulatory and policy-related barriers kept providers from practicing across state lines. Due to the pandemic and the rise of telemedicine, the next steps for the digital health field is to determine which procedures actually need to be performed in person.
She then offered some solutions to continue to improve the usage of digital healthcare for patients and providers: Teach trainees to utilize data and bring and normalize technology in the classrooms.
Zweifach also noted another approach to improving the efficiency of telehealth: bring in entrepreneurs and other innovators from startups to help separate unsuccessful health tools from successful ones. For example, Zweifach’s non-profit organization, NODE Health, brings together a network of providers, entrepreneurs, innovators and startups and provides them evidence-based digital health validation strategies. With that said, patient input is critical, and every strategy should be able to provide comprehensive care to meet patients’ complex needs. Zweifach highlighted Virta Health and Tia Women’s Health Clinic as leading companies that have successfully used data-driven, connected care with their patients.
FDA’s ‘Healthy Quality Systems’ by Courtney Lias, FDA
For diabetes medical devices, the Food and Drug Administration’s (FDA) role is to ensure a product is safe to use and that the benefits of devices outweigh the risks. Courtney Lias walked us through the accountability process that’s designed to keep people with diabetes safe and ensure devices maintain high standards. Lias stated that devices go through an independent assessment of validity, however, the device doesn’t have to be perfect; it’s all about comparing the benefits and risks.
The biggest takeaway from this session was the concept of recalls. It’s assumed when a product is under recall, that the product is inherently problematic. According to Lias, this isn’t the case. A prompt recall followed by an investigation and reports of adverse reactions are signs that the product is quite good and the manufacturer is taking the necessary steps to ensure safety. However, if a device manufacturer didn’t report on defects, complaints from customers and have a proper tracking system to collect data, that is a sign of an unhealthy product.
Most recalls are voluntary recalls initiated by the company when the company has to change something in their products. They may or may not include product removal but it is certainly a way to inform problems but rarely include market withdrawal. Recalls are simply an expected part of the process. Quality systems keep people safe.
Diabetes DIY in 2020 #WeAreNotWaiting: Global Projects Update by Wes Nordgren, Nightscout Foundation
The Nightscout family is a worldwide community in 35 countries around the globe with helping over 85,000 patients with diabetes.
Open APS (artificial pancreas system) is an algorithm that has super-advanced features, ”on a low carb diet with the G6 this new advanced features can make your type 1 diabetes (T1D) turn into T ”10” D because you only fuss with your type 1 diabetes every 10 days when you have to change your sensor,” according to Wes Nordgren from Nightscout. Among many other features of all these DIYAPS Open APS integrates with Nighscout, Android APS is compatible with a lot more pumps and has the capacity of providing profiles, time shifts and other features that the diabetes community was looking for.
Another important development is the enhancement of FreeAPS. The most important new feature is it can give micro bolus. This is currently under development.
Nightscout is developing DIY Edge. This is a new project in progress that requires two Eros Pods by Omnipod, one will provide insulin and another one will provide glucagon. The user would also wear one continuous glucose monitor (CGM) and use one algorithm. For athletes, this tool will be useful in managing blood glucose while competing.
To learn more about the Nightscout Project visit: http://www.nightscout.info/
New to DIY Looping and APS? Check out Beyond Type 1’s breakdown of it here: https://beyondtype1.org/the-guide-to-diy-looping/
New Product Demos Presented by the Diabetes Community:
HappyBob
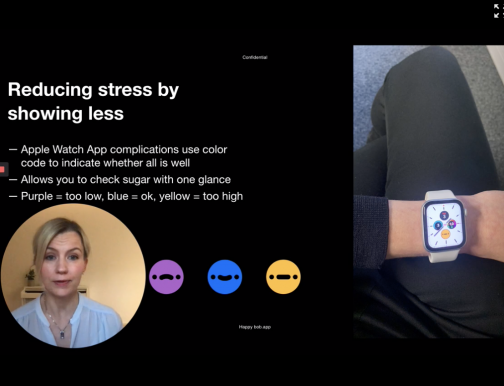
Happy Bob is an app created by a T1D mom, Jutta Haaramo. After her son was diagnosed they felt overwhelmed as a family. Diabetes imposes a heavy burden on both patients and caretakers. She wanted to create an app to reduce distress and anxiety that is usually a part of management. What would happen if each time you measured your blood glucose you felt something positive? What if it was somehow fun? How could we feel less scared?
Happy Bob makes the diabetes data collecting experiences less mentally exhausting by giving users an idea of their blood glucose levels using a color-coded system. Purple indicates low blood glucose, blue indicates in-range blood glucose and yellow indicates hyperglycemia.
This app uses a rewarding system to motivate and create positive experiences. This app uses CGM data and gives encouraging and personal advice to achieve happy numbers and blood glucose goals. This app can be linked to Dexcom, Nightscout and Google Health to import data. While data is still being collected, users of Happy Bob have seen improvements in Time-in-Range. Happy Bob is available for people with type 1 and type 2 diabetes and is suited for people of all ages.
Mariana’s Personal Note: This is amazing, I immediately downloaded the app and connected it to my Nightscout URL (you can also use Apple health), setting it up was easy and the app looks fantastic. Stars in the graph show blood sugar line, whenever blood sugar is between 4.0 mmol/L72 mg/dL and 10.0 mmol/L180 mg/dL, it’ll give a blue star, keeping blood sugar levels in the lower blue area (4.0-7.0 mmol/L72-126 mg/dL) will give double stars. Also, Bob app talks! So it might be a good positive rewarding system for some. Bob has an alter ego. I can’t wait to see what this means as he claims he’ll make me laugh, we’ll see.
Learn more about Happy Bob here: https://happybob.app/
Emmett App: A Parent’s Personal Diabetes Assistant

This is a voice-enabled app that leverages data collection from insulin pumps, wearables and CGMs to simplify management. HCW Emmet is a tool that helps people with diabetes and caregivers take better decisions. It was created by Dan Korelitz whose son, Emmett, lives with T1D and was diagnosed at 11 months old.
This is an algorithm that considers variables such as active nutrients, active insulin, averages and deviations from exercise and sleep patterns. The goal is to have the app eventually integrate with Alexa and other voice-enabled tools to work as an assistant that will provide individualized treatment recommendations.
Learn more about Emmett App here: https://www.youtube.com/watch?v=TeH0TJyJplo
LifePLUS Non-invasive CGM
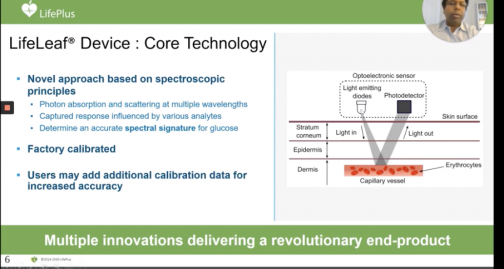
LifePlus presented its wearable “LifeLeaf,” as the world’s first non-invasive continuous glucose monitoring (CGM) smartwatch device. The device’s core technology uses spectroscopic principles to get an accurate spectral signature for blood glucose readings.
This device also tracks blood pressure, heart rate, respiration rate, cardiac arrhythmia, sleep apnea and oxygen saturation. With this information, the system’s AI generates personalized alerts and insights.
At this time, LifePLUS devices are not intended for the treatment and management of diabetes, hence why the first targeted groups are people with prediabetes and individuals without diabetes. The next iterations of this device will be targeted towards people with type 2 diabetes and then type 1 diabetes.
When compared to other continuous glucose monitors on the market, LifeLeaf products are designed to be significantly less expensive. The device is priced anywhere between $300-$600 and has an annual subscription.
Learn more about LifePlus here: https://www.lifeplus.ai/lifeleaf/
SugarBeat by Nemaura
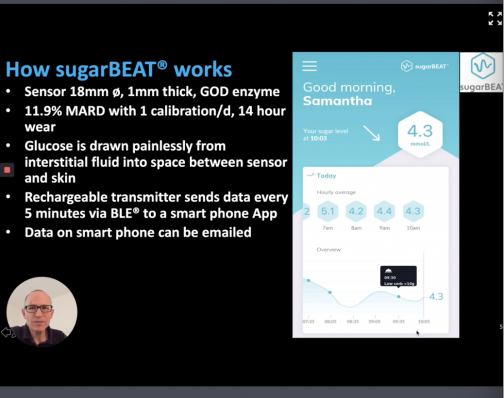
SugarBEAT® by Nemaura is another non-invasive continuous glucose monitor (CGM). It is a daily disposable adhesive skin-patch connected to a rechargeable transmitter, with an app that will display blood glucose readings every five minutes.
According to Nemaura “glucose molecules are drawn out of the interstitial fluid which naturally sits just below the top layer of skin, into a (sensing) chamber within a disposable adhesive patch. At the top of the chamber, the transmitter measures blood glucose and transmits the data to an app. This non-invasive CGM is part of BEAT a health subscription service providing one-on-one lifestyle coaching and behavior change recommendations are driven by personalized data provided by the device. Other features of the product include: no scanning needed, it’s environmentally friendly, it requires no battery and it’s affordable. Clinical studies have been completed, for type 2 and prediabetes as a first step.
With SugarBEAT, users change their adhesive patches every 14 hours, allowing them to wear it on non-consecutive days, as opposed to those who would need to wear other devices on a continuous basis. While the product isn’t available yet in the United States, the product is available for people with type 2, type 1 diabetes and prediabetes in Europe.
More info https://nemauramedical.com/sugarbeat/
Biolinq

Biolinq is a new needle-free application CGM. Although it is still an invasive CGM this is a smaller, silicon sensing device that claims to have a lower manufacturing cost. It is enabled by microarray technology with a microchip inside for measurement and data connectivity. No introducer needle just the surface of the skin to perform sensing. No lag time. It’s like putting on a Band-Aid and uses a normal adhesive. The sensor measures in micron millimeters. This will be an interoperable device although they are looking to have a sensor that performs better than the ones currently on the market.
Learn more about Biolinq here: https://www.biolinq.me/#Learn-More-Content
Patient-Led Research:
The Tidepool Loop Study Diana Naranjo, Stanford University
Naranjo presented an observational study that includes patients from the DIY community already using DIY Loop. Researchers wanted to understand the experience, efficacy and safety. Researchers saw an improvement in patients outcomes that were maintained and improved throughout the study and people were willing to recommend Loop after their positive experiences. People enjoyed having better time-in-range (TIR), decreased diabetes distress, improved sleep quality, decreased fear of hypoglycemia and meal flexibility. Some barriers reported were connectivity issues, difficult to set up, out of pocket costs and problem-solving.
A Deep Dive into Commercial Closed-Loop Systems in Real Life: a DiabetesMine study by Dana Lewis
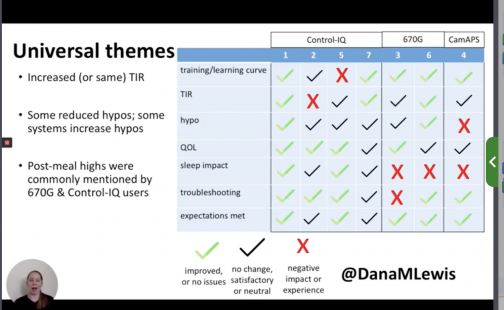
This study gave us insight into how people with type 1 and latent autoimmune diabetes in adults (LADA) diabetes perceived closed-loop systems in everyday life. In semi-structured phone interviews, seven users were surveyed about their experience with automated insulin delivery (AID) systems. The study sought a mix of experienced and newer AID users, using a mix of systems such as Tandem’s Control-IQ, Medtronic’s 670G and CamAPS FX.
With Control IQ, time-in-range increased by 30 percent with no change to hypos, less aggressive insulin dosage corrections, minimal troubleshooting and with a huge positive impact in their quality of life. Within 90 days, participants thought less about diabetes and had fewer disruptions in sleep. However, the frustrations recorded from this study were the lack of remote bolusing, monitoring on smartphones and plus two-hour warmup of CGM and doesn’t deal well with underestimated meals.
Those newer to CGM have a noticeable learning curve and were more likely to comment on the number of alarms and system alerts they received. The 670G users were more likely to describe connection and troubleshooting issues and CGM calibration issues, both of which impacted sleep. Post-meal glucose was also mentioned as a weak point in glycemic management with commercial AID systems.
Improving the User Experience for Diabetes Devices
Customer Support Challenges in an Interconnected World by Danielle Karsten, Tandem Diabetes Care
Control IQ is an advanced closed-loop system, designed to keep targets between 3.9-10.0 mmol/L70- 180 mg/dL. It adjusts insulin delivery based upon 30 minutes predicted CGM values including delivering automatic correction boluses as needed.
The unique advantage of Tandem’s update was that users could decide when they were ready to update their devices. When Control IQ launched, customers experienced long wait times while customer service representatives were struggling to answer customer questions about updating their Tandem software to use Control IQ.
Tandem analyzed the kind of calls they received in their call center so they quickly identified areas of opportunity especially in terms of information providing, instructions and user guides. Fortunately, Tandem was working on their site and the first week they leveraged their website so that people could search topics for which they required answers.
Recognizing the importance of having a variety of educational tools and resources, Tandem used its social media channels to provide education and awareness materials so people would find the information easily and they could reach a broader audience. In-person training is still important but people look for other channels to receive training. With their social media channels, Tandem developed educational videos, on how Control IQ worked, how to turn the system on and use the activity modes. The insulin pump company also used Facebook Lives to answer real-time questions by users and sent quick tech tips via email.
“Ditch the Manual: Visual, Interactive Diabetes Product Support” by Aimee Jose, Steady Health

Health literacy is critical in understanding new technology for people with diabetes. In this session, Aimee Jose from Steady Health discussed the ways device manufacturers can improve the user experience for their customers.
One of these methods is the use of videos, which are welcoming and enhance participation, especially if they use real people with real knowledge. For example, she showed a video of a young woman demonstrating how to insert the Omnipod system. Videos made by people are more comprehensible. Playlists are a way to organize educational videos that can help users and empower them to use new technology.
Another way to educate people with diabetes is to use Infographics. According to Jose, infographics are the new manuals. They can grab attention and can teach a new concept easily. However, infographics shouldn’t contain too much information. Interactive quizzes and modules are also a good way to promote learning. The looping community has experience in this area.
The Beyond Type 2 team is covering the American Diabetes Association’s 80th Scientific Sessions. For the latest breaking news at #ADA2020, click here. Follow us on our social media channels for live tweets of sessions and events, including the DiabetesMine D-Data ExChange.
Facebook – Beyond Type 2
Twitter – @BeyondType2