Semglee Receives FDA Approval as First Interchangeable Biosimilar Insulin
On July 28, 2021, the U.S. Food and Drug Administration (FDA) approved Semglee as the first interchangeable biosimilar insulin product for the treatment of type 1 and type 2 diabetes, developed by Mylan (now Viatris) and Biocon Ltd. This approval as an interchangeable biosimilar follows the official FDA approval of Semglee last June and is expected to increase the availability of insulin products while potentially lowering the cost of insulin for people with diabetes.
A biosimilar is a product that has no clinically meaningful differences to a biological product already approved by the FDA. Semglee is biosimilar to and interchangeable with Lantus, a long-acting insulin analog.
“The interchangeable biosimilar product can be expected to produce the same clinical result as the reference product,” Peter Stein, MD, director of CDER’s Office of New Drugs, says at the FDA Semglee press conference.
He adds that the risk of switching from the reference product to the biosimilar is not greater than the risk of using the reference product without switching. “It may be substituted at the pharmacy without the intervention of the healthcare provider, subject to state laws, similar to how generic drugs are substituted,” Stein says.
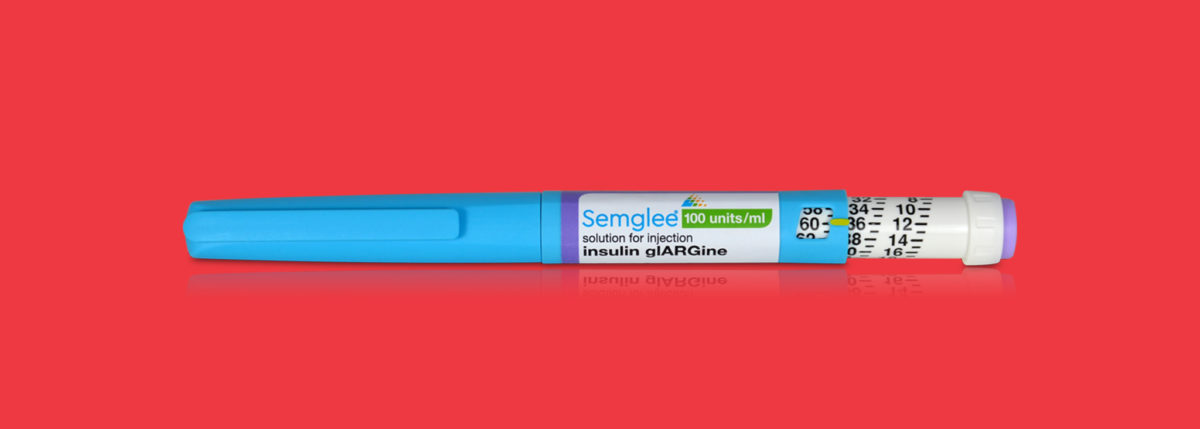
Semglee is administered subcutaneously once daily and is be offered in 10 mL vials and 3 mL prefilled pens. Similar to Lantus, the administration of Semglee will be dependent on the patient’s needs. Semglee is not recommended for treatment of hypoglycemic episodes or diabetic ketoacidosis.
Common side effects of insulin glargine products include edema, lipodystrophy, weight gain and allergic reactions, such as a rash, redness, pain at injection site and severe itching.
Pricing
Over the years, the list price of insulin has skyrocketed over 1,200 percent. From 1996 to 2019, a vial of Eli Lilly’s Humalog list price increased from $21 to $275. Biosimilars in the U.S. have typically launched with list prices 15 to 35 percent lower than reference products, potentially lowering the cost for some people with diabetes.
In contrast, Semglee’s initial list price was at a 65 percent discount, set at $98.65 per 10 mL vial and $147.98 per box of five (5) 3 mL pens. In contrast, the list price in 2019 for Lantus was $283.56 per one 10 mL vial and $425.31 for a box of five (5) 3 mL pens.
Additionally, Semglee has a copay program listed on their website where eligible patients with commercial health insurance can receive up to $75 off each 30-day prescription.
“This approval furthers FDA’s long standing commitment to support a competitive marketplace for biologic products, and ultimately empowers patients by helping increase access to safe, effective and high quality medications at potentially lower cost, which millions of Americans take each day to maintain stable blood glucose,” Stein says. “Our hope and Congress’s intent is that the availability of biosimilar and interchangeable products will help bring down these costs to market competition.”
The list price of Semglee is still significantly higher than what people pay for insulin using commercial insurance. It is also more expensive than analog insulin available through co-pay cards and patient assistance programs. However, for those who may not qualify or need insulin immediately, a lower list price at the pharmacy counter could go a long way.
This content mentions Lilly, Sanofi and Viatris, active partners of Beyond Type 1.
News coverage by the Beyond Type 1 team is operated independently from any content partnerships. Beyond Type 1 maintains full editorial control of all content published on our platforms.