Diabetes news
2018-12-11
Rezdiffra Approved for MASH—A Diabetes-related Liver Disease
On March 14, 2024, the U.S. Food and Drug Administration approved resmetirom (Rezdiffra)—the first drug for a diabetes-related liver disease known as MASH. The approval makes way for the first and o...MORE

Dexcom Introduces Direct-to-Apple-Watch Feature for its G7 CGM
Dexcom recently introduced a direct-to-Apple-Watch feature for its G7 continuous glucose monitor (CGM) users. This means G7 will be the only CGM system that can directly connect to Apple Watch—witho...MORE
Payment Assistance and Savings Program Extended for the Eversense E3 CGM
Ascensia Diabetes Care and Senseonics have extended their Eversense Payment Assistance and Simple Savings (PASS) program—aimed to help people in the U.S. more affordably access the Eversense E3 cont...MORE

Stelo Becomes the First Over-the-Counter Glucose Biosensor in the U.S.
On March 5, 2024, Dexcom, Inc. announced that the FDA has cleared Stelo to become the first over-the-counter glucose biosensor in the United States. Stelo, which is indicated for persons 18 and older,...MORE

ADA Standards of Care 2024: So What’s New?
Diabetes research, technology, and treatments are rapidly changing. Here's what you need to know about the 2024 Standards of Care.MORE
Zepbound: A New Medication for Chronic Weight Management
Learn more about Zepbound, the latest medication to receive FDA approval for the treatment of obesity or weight-related medical problems.MORE

Benefits of Using a Dexcom G7 CGM During Pregnancy
Managing diabetes during pregnancy can be tough, but Dexcom Chief Operating Officer Jake Leach shares how a CGM can help.MORE

Video: Type 2 Diabetes Virtual Wellness Summit
🌟 Beyond Type 2’s Virtual Summit! 🌟 Watch the recording of our first Type 2 Diabetes Virtual Summit, an event that’s one of the industry’s first for people impacted by type 2 d...MORE

GoodRx Announces New Way for People to Access Lantus for $35
GoodRx partnered with Sanofi to offer $35 insulin coupons to all, regardless of their insurance status, for use at pharmacies nationwide. MORE

New Program Recognizes Leaders In Diabetes Care
Received stellar diabetes care? Encourage your providers to apply to be recognized as leaders in diabetes care.MORE

Ketogenic Diet for Kids with Diabetes Carries Risk
The American Academy of Pediatrics warns against a ketogenic diet, a popular low-carbohydrate diet, for children with diabetes.MORE

Dexcom ONE Now Available in France
Dexcom ONE, a continuous glucose monitoring (CGM) system, is now available for people with diabetes in France.MORE
Beyond Type 2 Expands to Provide Resources to Canada
Canadians can now share their stories, get connected to the community and find critical resources on all things type 2 diabetes. MORE

New Pfizer Vaccine Available Fall 2023
The new COVID vaccine shot will soon be available for fall. This article will detail everything you need to know to protect yourself.MORE

Project IMPACT: Bringing CGM Access to a Pharmacy Near You
Interview with Robert E. Nichols, PharmD, BCPS, Clinical Pharmacist, who is participating in the pilot program for better CGM access. MORE

I Lost Medicaid—Now What?
In May 2023, millions of people faced losing Medicaid coverage—and fast. Here's what to do if you're impacted.MORE

Beyond Type Run Marathon Team Reaches New Milestones in 2023
Beyond Type Run, the stereotype-smashing team of runners proves anybody living with diabetes can go the distance despite their diagnosis.MORE

Mark Cuban’s Cost Plus Drugs Offers New SGLT-2 Medication for Type 2 Diabetes
Brenzavvy is the latest SGLT-2 medication for type 2 diabetes to be added to Mark Cuban’s Cost Plus Drug company’s product lineup, helping improve the affordability of the drug.MORE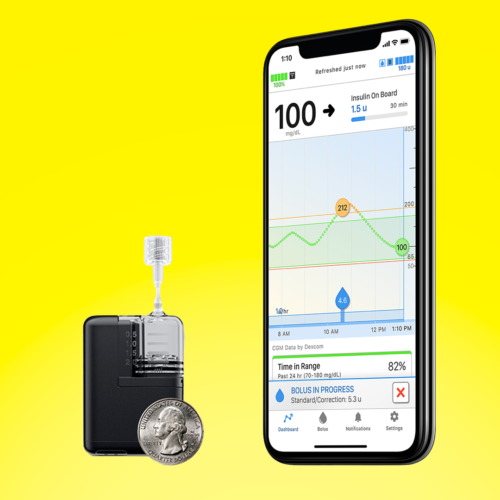

Tandem’s Mobi Approved by the FDA
Tandem’s newest insulin pump, Mobi, was approved by the FDA for people with diabetes age six and up. Here's what you need to know!MORE

A New Medication Targeting Three Hormones
Retatrutide, a new medication, is showing promising results for the treatment of several diseases including type 2 diabetes. MORE

A Year in Review: Advancements in Diabetes Tech + Research
This session reviewed the advances and discoveries in research and technology for people living with diabetes over the past year.MORE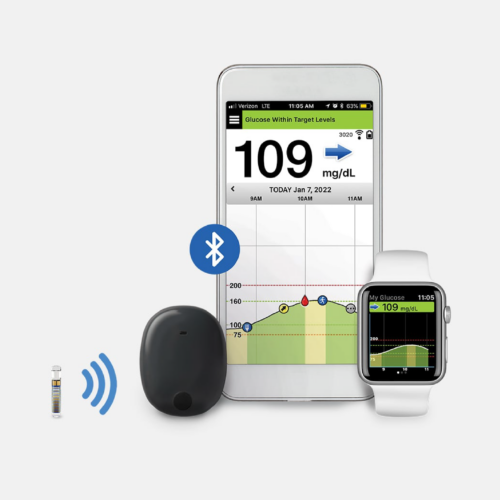
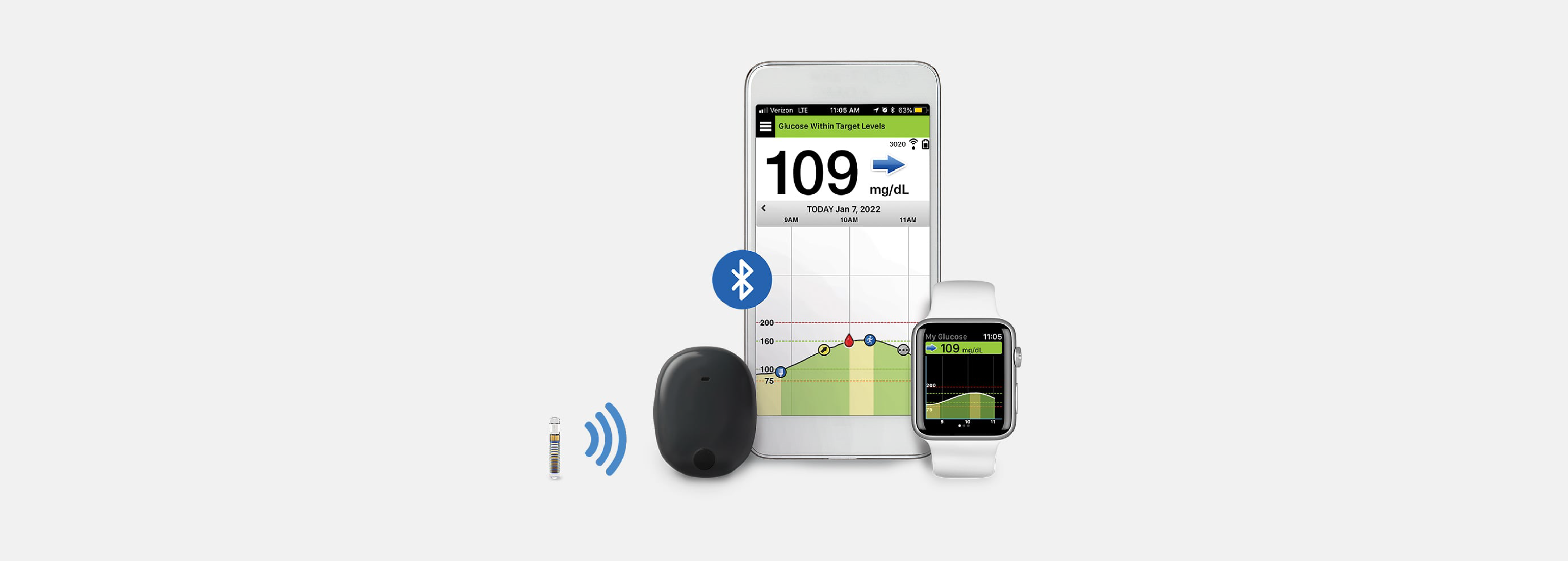
Senseonics + UnitedHealthcare Expanding CGM Access
Senseonics announced that UnitedHealthCare will now cover their Eversense E3 implantable CGM, starting July 1, 2023.MORE

Omnipod GO Receives FDA Approval for Adults with Type 2 Diabetes
This FDA approval marks the first-of-its-kind of a basal-only tubeless insulin pod for adults with type 2 diabetes. MORE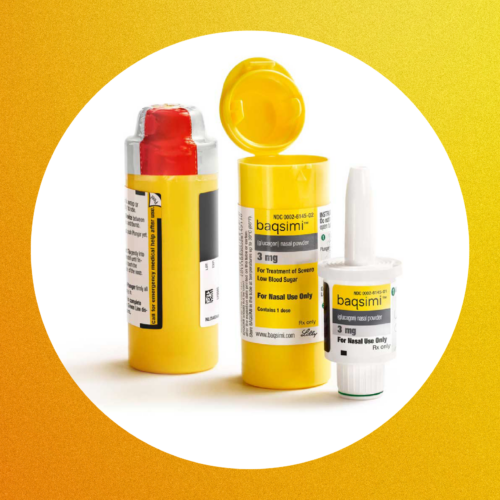

Eli Lilly’s Baqsimi Acquired by Amphastar: What This Means for People With Diabetes
Eli Lilly’s nasal glucagon, Baqsimi, has been acquired by Amphastar. Here’s what you should know about the change and its significance.MORE
Mark Cuban’s Cost Plus Drugs Makes Diabetes Products More Affordable
Mark Cuban's Cost Plus Drugs Company just announced that they offer Janssen's Invokana, Invokamet and Invokamet XR for type 2 diabetes.MORE

California Partners with Civica Rx to Make More Affordable Insulin
California has signed a contract with Civica Rx to make more affordable insulin for Americans with diabetes. Here’s what you should know about their partnership, CalRx.MORE

Sanofi to Slash Price of Lantus by 78%, Cap Out-of-Pocket Costs to $35
Sanofi has capped monthly out-of-pocket costs for Lantus to $35 for all with commercial insurance and will cut the price of Lantus by 78%, effective January 1, 2024. MORE

Novo Nordisk to Slash Insulin Prices by over 65%
Novo Nordisk announced that starting January 1st, 2024 they will be lowering the list price of several of their insulins by 65-70%.MORE

$25 List Price + $35 Copay Caps: Lilly’s New Insulin Cost Reductions
On March 1, 2023, insulin manufacturer Lilly announced expanded insulin cost-saving measures for people with and without health insurance coverage.MORE

This New App is Designed to Make Tracking Your Insulin Easier
If you use Lilly brand insulin, this new connected platform is designed to make tracking your diabetes data a lot easier. MORE

Explore the Beyond Type 2 Community
Join the Beyond Type 2 Community, a safe space for those with type 2 diabetes to connect, share experiences, and get questions answered.MORE

Say Hello to BeyondType2.org in French, German and Italian!
BeyondType2.org now offers critical resources in five languages for people living with and impacted by type 2 diabetes.MORE

Congress Wants You to Know About These New, Cheaper Insulins
This legislation aims to help you and your doctor understand the potential benefits of cost-saving biosimiliar drugs and how to access them.MORE
Insulin Prices Are Rising Unchecked. New Biosimilar Insulins May Cost You Less.
You may soon be able to substitute newer, cheaper insulins that work exactly the same as your current prescription.MORE
Lilly to Discontinue Old School Glucagon Emergency Kit at End of 2022
With several modern and ready-to-use glucagon options available, Lilly will discontinue the old, red glucagon emergency kit in all markets. MORE
UnitedHealthcare Announces $0 Copay on Insulin, Glucagon + More, Beginning in 2023
UnitedHealthcare announced a $0 copay on insulin, glucagon and other drugs starting in 2023. Here's what that could mean for UHC members.MORE

New Program Offers $35 Monthly Copays for Sanofi Insulins
Sanofi has announced a $35 per month insulin copay program for uninsured individuals in the U.S, effective July 1, 2022.MORE

Type 2 Diabetes in Children + Teens: A History of Care + its Challenges
Understanding the unique challenges for young people with type 2 diabetes and their families can improve care and education. MORE

Year in Review: The Biggest News in Diabetes Clinical Science
Highlights from the last year of diabetes research and treatment include new biosimilar insulins and updated screening and diagnosis guidelines.MORE

Research: Practical Use of Closed-Loop Insulin Pumps
Closed-loop insulin pump technology is a game-changer for those taking insulin. Here are essential tips to using them in day-to-day life.MORE

Weight Management + Type 2 Diabetes: Using Compassion, Medicine and Surgery
Weight loss has several benefits for people with type 2 diabetes. However, it isn’t always easy to achieve through only lifestyle changes.MORE

The Future of Insulin—Will It Be Affordable for the People Who Need It?
Research is showing promising advancements with new weekly oral and injectable insulins—but will they actually be affordable?MORE

Diabetes + Dementia: Using Nasal Insulin to Improve Brain Function
With rising rates of obesity in both type 1 and type 2 diabetes, understanding the impact on long-term brain health and function is critical.MORE

Researchers Talk: Past, Present and Future of Pancreas Transplants
Are pancreas transplants the way of the future for people with diabetes? Here’s what you should know about the procedure.MORE

Not All Net Carbs and Artificial Sweeteners are Alike
Experts discuss the complexities of how net carbs and artificial sweeteners affect everyone with diabetes differently. MORE

Young Adults with T1D + T2D Need Personalized Care
By understanding the unique challenges of early adulthood, providers can empower young adults with type 1 and type 2 diabetes.MORE

When is the Right Time to Eat with Diabetes?
Experts discuss if there’s an optimal way to time your meals and the common “3 meals and 3 snacks per day” rule. MORE

Type 2 Diabetes in Children + Teens: A History of Care + its Challenges
Understanding the unique challenges for young people with type 2 diabetes and their families can improve care and education. MORE

Reaching Everyone—Health Disparities in Physical Activity
People living with diabetes are told to just exercise more to better manage their diabetes. Here is why it's not that simple. MORE

Race, Racism, and Diabetes Research
Diabetes care and research is still drastically impacted by racist practices. It’s time to call the racism out. MORE

Challenges + Solutions for Underserved Communities in East Los Angeles
People living with diabetes in East L.A. face many challenges in getting the care they need. Is Blue Circle Health a potential solution?MORE

Addressing Social and Structural Health Inequities in Diabetes Care
To address long-standing health inequities, providers and health systems must engage with barriers to high-quality diabetes care.MORE

The Negative Impact of Diabetes Stigma: “Words create reality”
Key behavioral science research proves the impact of attitudes and words on people living with diabetes and why those stigmas must change.MORE

Weight Bias + Diabetes: “Stigma does not motivate anyone”
In this coverage, we share research highlighting how weight bias affects people with diabetes and what can be done to change public attitudes.MORE

Mounjaro: Its Powerful Potential to Treat Type 2 Diabetes + Obesity
Experts discuss the impressive impact and future potential of using Mounjaro (tirzepatide) to treat obesity and type 2 diabetes.MORE

Diabetes Standards of Care Updated for Preventing or Delaying Type 2 Diabetes
The ADA has updated the standards of care to include recommendations that focus on preventing or delaying type 2 diabetes and its associated complications.MORE

Another Reminder that Racial/Ethnic Inequality Exists in Diabetes Care
Despite the variety of diabetes medications and technology, race and ethnicity still weigh heavily on a person’s access to these life-saving tools.MORE

Access to Insulin: In the United States
Despite the growing variety of insulin and technology, access to this life-saving drug continues to be a dire problem in the United States.MORE

Access to Insulin: In Low-Income Countries
In many countries, a person with diabetes may walk for four days to get a vial of short-acting insulin and seven to 10 days for long-acting.MORE
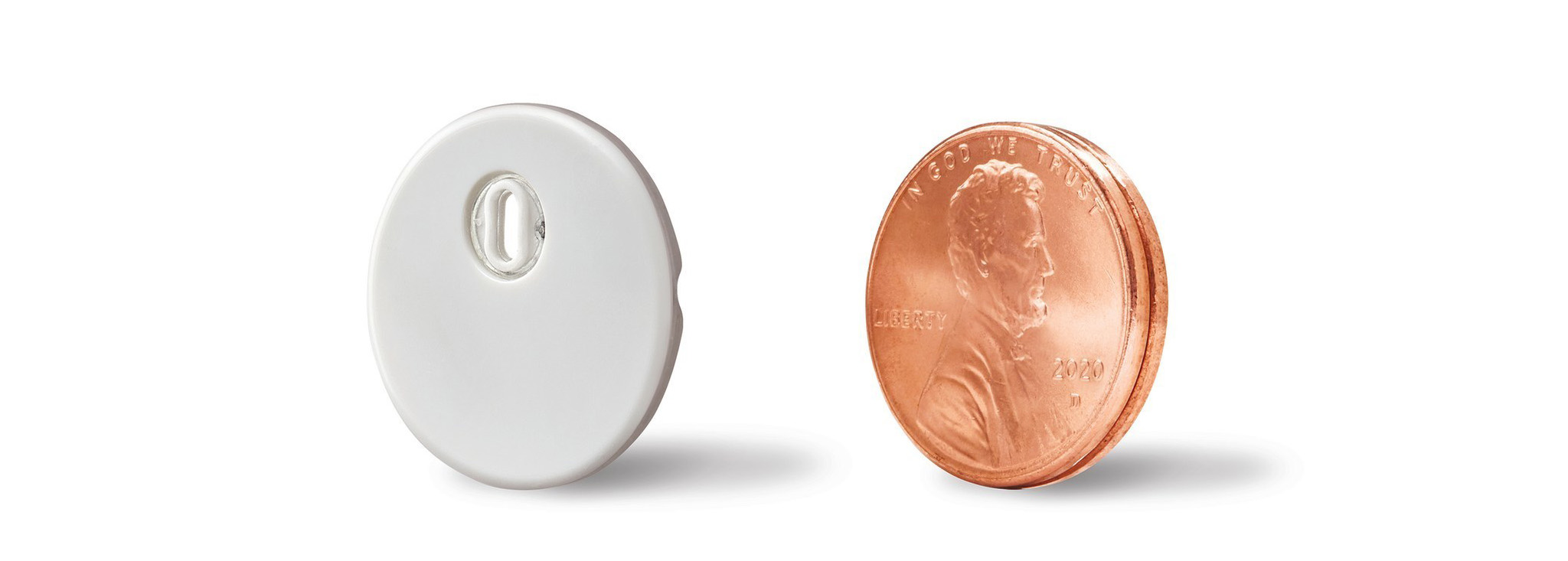
Abbott’s Freestyle Libre 3 Receives FDA Approval
The Freestyle Libre 3, the world's smallest continuous glucose monitor, offering several improvements and updates from the Libre 2, has received FDA approval.MORE

Research Suggests Changing Treatment Approach for Type 2 Diabetes
With more medication and technology available for treating type 2 diabetes, it's about time more providers make better use of them. MORE

Mounjaro Shows Multiple Benefits for Type 2 Diabetes Management
New, FDA-approved Mounjaro shows promising results for improving several aspects of type 2 diabetes management. MORE

FDA Approves Lilly’s Mounjaro: First of its Kind Medication for Treatment of Type 2 Diabetes
The FDA approved Mounjaro from Lilly, the first GIP/ GLP-1 receptor agonist to treat people with type 2 diabetes. MORE

Promising New Drug Leads to 22.5% Weight Loss in Adults Living with Obesity
The latest study on a new drug is making headlines for its impressive ability to help patients lose weight.MORE

Despite Universal Healthcare, Diabetes Disparities Persists in the United Kingdom
To address the racial and economic divide in diabetes technology use, a top diabetes specialist urges a change in pump and CGM training.MORE

ATTD 2022 Coverage: Advanced Technologies + Treatments for Diabetes
ATTD's annual conference covers the latest in diabetes technology and treatment. This year's event is in Barcelona from April 27-30, 2022. MORE

Coverage of Dexcom G6 CGM Now Improved with TRICARE
TRICARE healthcare members can now receive the Dexcom G6 continuous glucose monitor (CGM) under pharmacy benefits.MORE

The Rise of Prediabetes Diagnoses
There has been an increase in the number of people diagnosed with prediabetes. We spoke with Dr. Mayer B. Davidson to learn why.MORE

Beyond Type 1 Names Deborah Dugan as New CEO
Global diabetes nonprofit Beyond Type 1 has announced the appointment of its new CEO Deborah Dugan, former CEO of (RED).MORE
A Second COVID-19 Booster Dose + The Latest Omicron Variant
Keeping up with the latest COVID-19 shot and variant news can be difficult. Here’s what you should know about Omicron XE and a second booster!MORE

A1c Test Results Are More Likely To Be Inaccurate for People Of Color–Here’s Why That Matters
A1c is one of the go-to tests for monitoring blood sugar, yet for people of African, South and Southeast Asian and Mediterranean ancestry, there’s a higher chance it’s not accurate.MORE

The Affordable Insulin Now Act: A Step Toward Federal Legislation
The Affordable Insulin Now Act, if passed in its current form, could help bring down the cost of insulin for millions of insured people with diabetes.MORE

FreeStyle Libre 2 Now More Easily Accessible for Military Members Through TRICARE
Military members with TRICARE now have easier access to Abbott’s FreeStyle Libre 2 continuous glucose monitor (CGM).MORE

Civica Rx Aims to Bring $30 Insulin to Market in 2024
Launching to market in 2024, Civica Rx aims to make insulin available for no more than $30 per vial, regardless of insurance status.MORE

COVID-19 Pushes FDA Approval of Breakthrough Device Designation for Dexcom CGM in Hospital Settings
The FDA has granted Breakthrough Device Designation for Dexcom CGM in hospital settings after the pandemic highlighted essential care needs for patients with diabetes.MORE

If You Take Insulin for Type 2 Diabetes, Your Doctor Should Also Prescribe This…
If you take insulin as a person with type 2 diabetes, your healthcare provider should prescribe emergency glucagon, too. Here’s why…MORE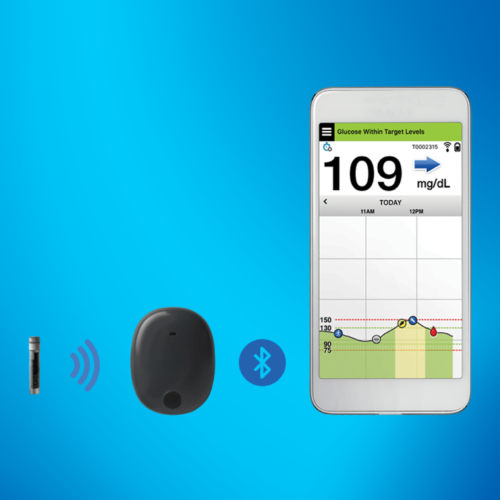
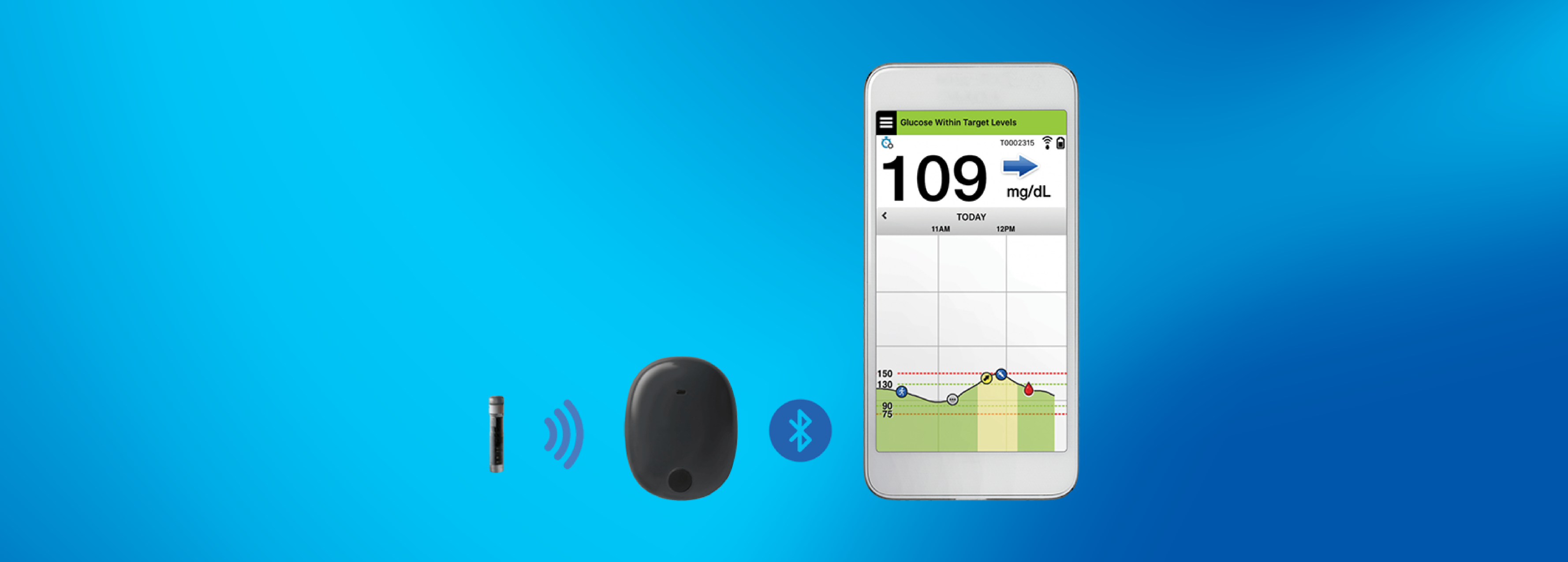
Eversense E3 Continuous Glucose Monitor Gains FDA Approval, Offers Extended Wear-Time
The Eversense E3 continuous glucose monitor (CGM) is an exciting advancement for the diabetes community, with improved wear-time and accuracy.MORE

Inside the Drug Pricing Investigation from the House Oversight and Reform Committee
The results of the most recent report from the committee make things clear for the United States—the need for access to affordable healthcare is direr than ever.MORE

Mark Cuban’s New Online Pharmacy Provides Affordable Access to Some Common Prescription Drugs
Mark Cuban's latest investment in the pharmaceutical industry offers common generic prescription medications (not including insulin) at a fraction of the cost.MORE
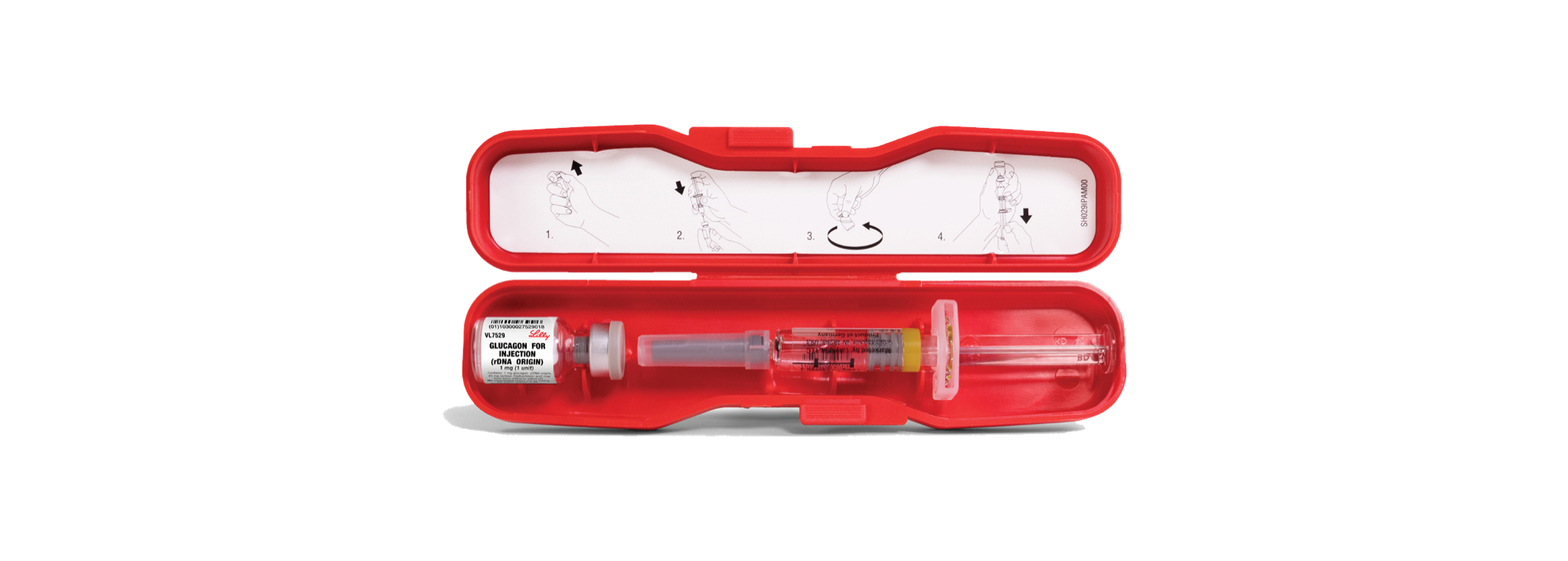
Lilly Issues Voluntary Nationwide Recall of One Lot of Glucagon Emergency Kit
Lilly Diabetes announced the voluntary recall of lot D239382D, Expiration April 2022 of Glucagon Emergency Kits.MORE

The Latinx Diabetes Initiative Provides Culturally Tailored Care To Latinx Patients With Diabetes
Andreina Millan Ferro is centering culturally sensitive diabetes care for Latinx patients with diabetes at the Latinx Diabetes Initiative.MORE
All About Gvoke HypoPen®
Anyone who takes insulin and other medications that cause low blood sugar should know about the premixed insulin Gvoke HypoPen.MORE

Empowering Youth Through Diabetes Education In Mexico
Yarishdy Mora is the director of the Youth Health Program for Project HOPE in Mexico. She creates platforms that help youth learn about diabetes. MORE

Overweight Adults to Now Be Screened for Type 2 Diabetes At 35
The U.S. Preventive Services Task Force now recommends overweight and obese adults to get screened for Type 2 diabetes at the age of 35.MORE

The 2021 Association of Diabetes Care and Education Specialists (ADCES) Virtual Conference
The Beyond Type 2 team covered the virtual 2021 ADCES Conference, covering the latest in diabetes education for the T2D community.MORE

Pandemic Homelessness Disproportionately Impacts People With Diabetes
An American Diabetes Association report found that pandemic homelessness hit people with diabetes 48 times harder.MORE

Youth with T2D Experience Long-Term Complications By Young Adulthood
Type 2 diabetes cases are rising in children and we're starting to learn they experience long-term complications by young adulthood. MORE
Abbott’s FreeStyle Libre 2 App Receives FDA Clearance
On August 2, 2021, the FDA approved the FreeStyle Libre 2 app for adults and children with diabetes, allowing user to access glucose readings directly from their iPhone.MORE
FDA Approves Senza®, Nevro’s High Frequency Spinal Cord Stimulation Therapy For PDN
The Senza System has been approved by the FDA for the treatment of chronic pain associated with painful diabetic neuropathy. MORE

Semglee Receives FDA Approval as First Interchangeable Biosimilar Insulin
Semglee becomes the first interchangeable biosimilar product for the treatment of Type 1 and Type 2 diabetes.MORE

Providing Culturally Sensitive LGBTQ+ Diabetes Health Care
Culturally sensitive LGBTQ+ diabetes health care can decrease stigma and advance diabetes care access for the LGBTQ community. MORE
FDA Approves Kerendia For The Treatment Of Type 2 Diabetes
Kerendia will be available in the U.S. immediately for the treatment of chronic kidney disease associated with Type 2 diabetes.MORE

Research on Pancreas Transplants in Type 1 and Type 2 Diabetes
Pancreas transplants are not an easy solution to type 1 or type 2 diabetes, but for some, they may be life-saving.MORE

Healthcare Providers Will No Longer Be Allowed to Issue “Surprise” Bills
Editor’s Note: People who take insulin require consistently affordable and predictable sources of insulin at all times. If you or a loved one are struggling to afford or access insulin, you can buil...MORE

Walmart Launches Lower-Cost Analog Insulin: ReliOn NovoLog
ReliOn NovoLog will be available at Walmart and Sam's Club pharmacies for $72.88 per vial and $85.88 per box of five FlexPens.MORE

Effects of Medicaid Coverage on the Diabetes Prevention Program in California
Obidiugwu K. Duru presents at the 81st ADA conference on how medicaid impacts the California Diabetes Prevention Program.MORE

News from DiabetesMine™ D-Data Exchange 2021
D-Data is the event, where attendees can discover, learn about, and try demos of new diabetes tech. Here is a recap of the sessions we attended at D-Data 2021.MORE
Zegalogue® Is Now Commercially Available in the United States
Zegalogue®, also known as dasiglucagon, is now commercially available in the U.S. for the treatment of severe hypoglycemia.MORE

Breaking News from ADA 2021
Breaking news from the biggest diabetes conference of the year - the American Diabetes Association's 81st Scientific Sessions.MORE

American Diabetes Association’s 81st Annual Scientific Sessions: #ADA2021
The biggest diabetes conference of the year is virtual for the second year + Beyond Type 2 is reporting live! Keep up with our coverage here. MORE




